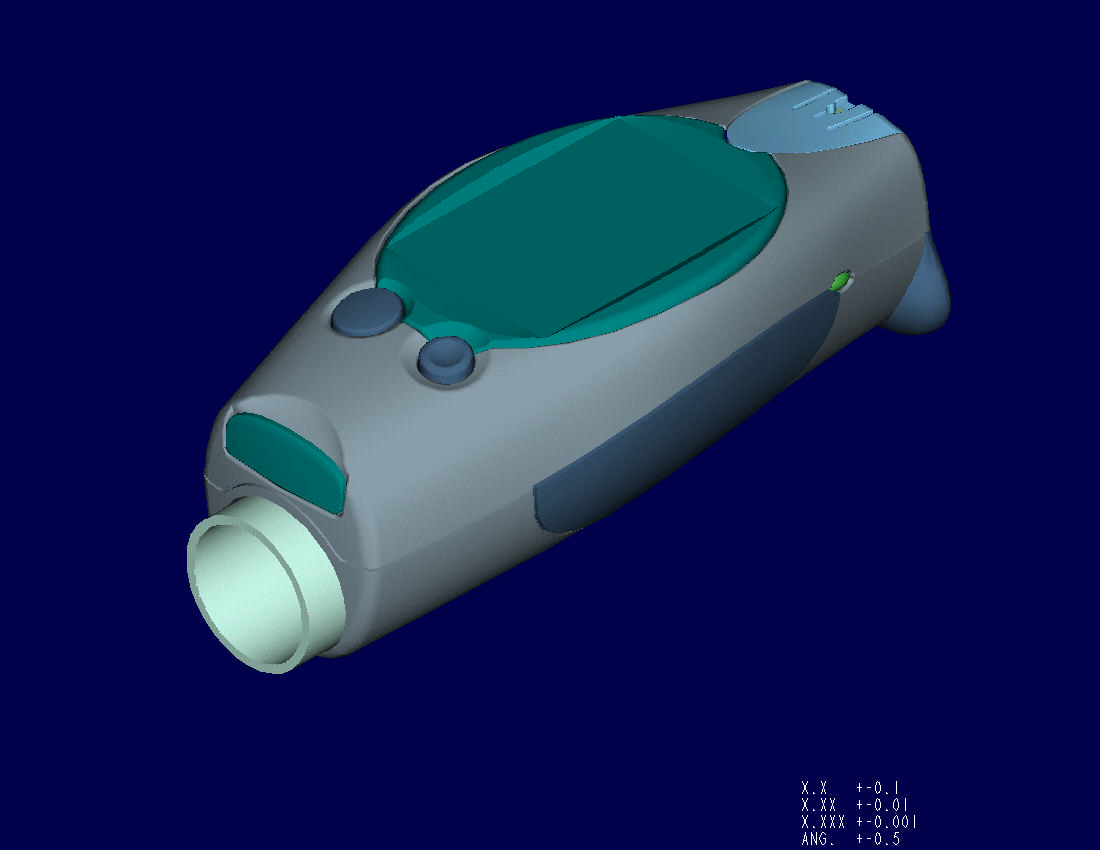
Summary:
- A mid-size early-stage
medical device company concentrating on blood glucose meter with
integral lancing device for alternate site (non-fingertip) testing.
- First generation
product already on the market.
- Acquired by Roche
Diagnostics Diabetes Care in 2001.
Major Accomplishments:
- Lead Mechanical
Engineer responsible for the 2nd generation meter.
- Generated
manufacturable designs from the industrial design surface models and
worked with Phillips Plastics to get the tooling into a
production-ready state.
- Performed all design
changes to the heavily surfaced Pro/E files and documented the changes.
- Learned and followed
company quality procedures.
- Developed DVT
protocols.
- Designed and
documented all mechanical changes relating to the component parts and
the circuit board.
- Drove the development
a new electrical connector used for test strip lot coding to replace
the problematic design in the 1st generation meter. Responsible for new
product introduction (NPI) of a new blood expression tip in
partnership with a Japanese OEM customer and the China-based contract
manufacturer.
- Led the selection,
justification, adoption, and implementation of a new product data
management system for Pro/Engineer data.
Relevant Expertise:
- FDA 21 CFR § 820
- Pro/Engineer software
- Product Data Management
- Injection molding
- Mechanism design and
analysis
- Electronics packaging
and interconnection
- Documentation and
document control
- Display technology
|