Summary:
- Lifescan is the
diabetes care division of Johnson and Johnson.
- Joined as a contract
mechanical engineer in the sustaining engineering group with R&D.
- Lifescan is
consolidating its operations and closing R&D in Milpitas as of
December 2010.
Major Accomplishments:
- Core team
member and mechanical resource on a project to improve the stability of
the time/date setting
on the Ultra Mini blood glucose meter.
- Worked with an
international team to research failure modes, analyze improvement
options, and recommended optimum structural improvements to a
meter/pump controller having field durability issues.
- Supported the launch
and subsequent failure analysis of a new lancing device. Collaborated
with Lifescan and vendor teams to develop recommendations for several
product improvements.
Relevant Expertise:
- FDA 21 CFR § 820
- Pro/Engineer software
- Windchill software
- Solidworks software
- Enable document
management system
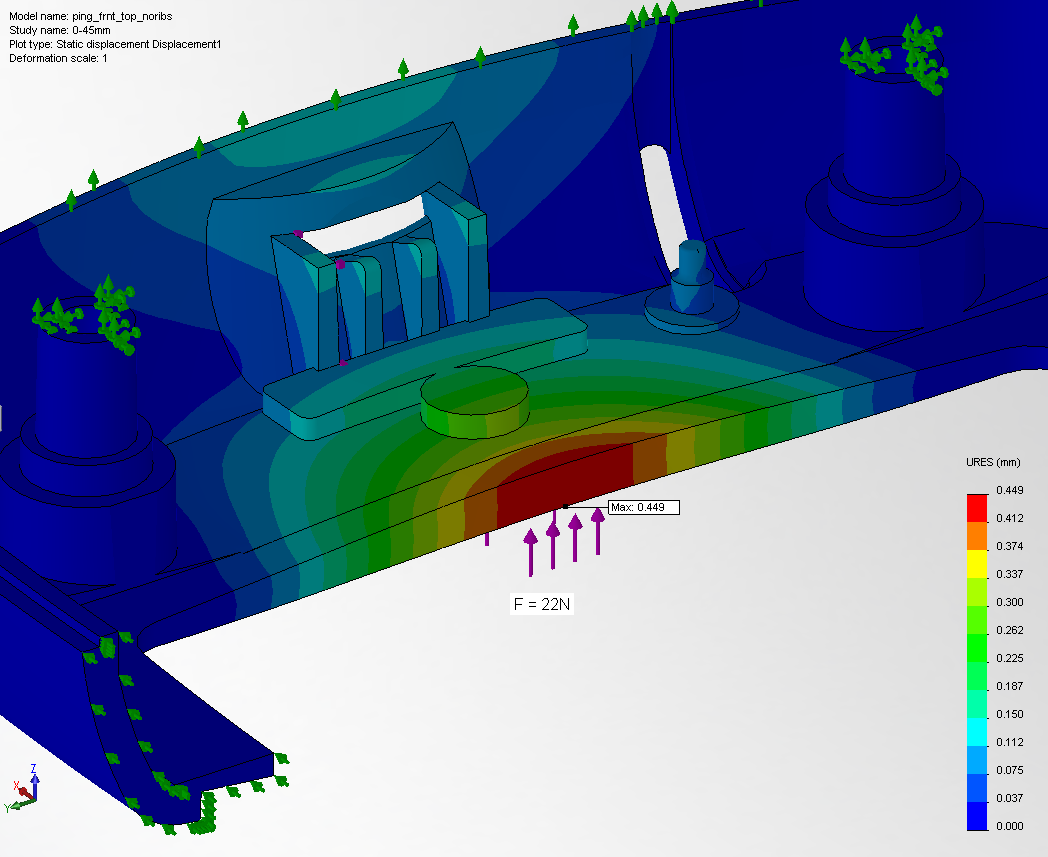
- Finite element analysis
- Failure analysis
- Mechanism analysis
- Injection molding
- Documentation and
document control
|